Roche announced that Erivedge (vismodegib) capsule was approved by the U.S. Food and Drug Administration (FDA) for adults with a type of skin cancer, called basal cell carcinoma (BCC), that has spread to other parts of the body or that has come back after surgery or that their healthcare provider decides cannot be treated with surgery or radiation.
Erivedge is the first FDA-approved medicine for people with advanced forms of the most common skin cancer. It is a capsule that is taken orally once-a-day.
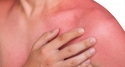
Basal cell carcinoma is generally considered curable if the cancer is restricted to a small area of the skin. However, in rare cases, lesions can become disfiguring and invade surrounding tissue (locally advanced) or spread to other parts of the body (metastasize). In these cases of advanced BCC, the disease cannot be effectively treated with surgery or radiation. Advanced BCC often results in severe deformity or loss of function of affected organs.
“Today’s approval provides a new treatment for people with advanced basal cell carcinoma who, until now, had no approved medicines to help shrink disfiguring or potentially life-threatening lesions,” said Hal Barron, M.D., Chief Medical Officer and Head, Global Product Development. “We are pleased that in the last six months we have been able to provide two new medicines for different types of advanced skin cancer to people who previously had few or no treatment options.”
Erivedge will be available in the United States within one to two weeks of approval and will be distributed through specialty pharmacies. Roche has also submitted a marketing authorisation application (MAA) for Erivedge in the European Union (EU). In order to provide people with advanced BCC who live outside of the United States access to Erivedge while Roche pursues marketing authorisation world-wide, the company is conducting a phase II safety study in the EU and other countries that is enrolling patients with advanced BCC.
Erivedge efficacy in advanced basal cell carcinoma
The FDA approval of Erivedge is based on results from ERIVANCE BCC (SHH4476g), a pivotal international, single-arm, multicenter, two-cohort, open-label, phase II study that enrolled 104 patients with advanced BCC, including locally advanced BCC (71) and metastatic BCC (33).
The study showed Erivedge shrank lesions (objective response rate, or ORR) in 43 percent (27/63) of patients with locally advanced BCC and 30 percent of patients (10/33) with metastatic BCC, as assessed by independent review, the primary endpoint of the study. The median duration of response was 7.6 months.
Patients with locally advanced BCC had lesions that recurred after surgery, were not candidates for surgery (inoperable, or for whom surgery would result in substantial deformity), recurred after radiotherapy or were not candidates for radiotherapy (radiotherapy was contraindicated or inappropriate). Study participants received 150mg of Erivedge orally, once daily until disease progression or unacceptable toxicity.
Safety information for Erivedge
The most common side effects of Erivedge are muscle spasms, hair loss, change in how things taste or loss of taste, weight loss, tiredness, nausea, diarrhoea, decreased appetite, constipation, vomitting and joint aches. Other side effects may include missed monthly periods in females who can become pregnant, low levels of sodium in the blood, low potassium levels, and a higher than normal blood level of urea or other nitrogen containing compounds in the blood. Patients should tell their healthcare provider if they have any side effect that bothers them or that does not go away.
Please see the full prescribing information for Erivedge, including the Boxed WARNING and Medication Guide at http://www.Erivedge.com.
About basal cell carcinoma and the Hedgehog pathway
BCC is the most common type of skin cancer in Europe, Australia and the United States. In advanced BCC, if the disease is left untreated or recurs in the same location after surgery or radiotherapy, it may advance further into surrounding areas such as sensory organs (ears, nose and eyes), bone, or other tissues. Depending on the location of the lesion, some cases of advanced BCC can be disfiguring, and treatment with surgery or radiation can lead to the loss of sensory organs and their functions such as eyesight or hearing.
The Hedgehog signaling pathway plays an important role in regulating proper growth and development in the early stages of life and becomes less active in adults. Abnormal Hedgehog signaling is implicated in more than 90 percent of BCC cases.
About Erivedge (vismodegib)
Erivedge is an oral medicine designed to selectively inhibit abnormal signaling in the Hedgehog pathway, which is an underlying molecular driver of BCC. Roche and Genentech are also evaluating Erivedge in a Phase II trial in people with operable forms of BCC.
Roche is developing Erivedge under a collaboration agreement with Curis, Inc. Erivedge was discovered by Genentech and jointly validated by Genentech and Curis through a series of preclinical studies. Through this collaboration, Genentech (United States), Roche (ex-United States excluding Japan and Korea) and Chugai Pharmaceuticals (Japan and Korea) are responsible for the clinical development and commercialisation of Erivedge. Curis is eligible to receive cash payments upon the successful achievement of specified clinical development and regulatory approval milestones, as well as royalties upon commercialisation of Erivedge.
For more information about Erivedge, go to http://www.Erivedge.com