(RxWiki News) The US Food and Drug Administration (FDA) has approved two medications to treat children with hepatitis C.
These new approvals are for Sovaldi (sofosbuvir) and Harvoni (ledipasvir and sofosbuvir). The FDA approved them to treat children between the ages of 12 and 17 who have hepatitis C.
Both of these medications were previously approved to treat adults with hepatitis C. Now, they're the first direct-acting antiviral medications that have been approved to treat children and adolescents with the disease.
Harvoni was approved to treat children who are 12 years old or older or who weigh at least 77 pounds. It is approved for hepatitis C virus types 1, 4, 5 or 6 in patients without cirrhosis (liver disease) or with mild liver disease.
Sovaldi was approved to be given with ribavirin to children who are 12 years old or older or who weigh at least 77 pounds. This medication is approved for hepatitis C virus types 2 or 3 without cirrhosis or with mild liver disease.
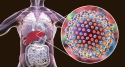
An estimated 2.7 to 3.9 million Americans have chronic hepatitis C virus, according to the Centers for Disease Control and Prevention. Hepatitis C virus leads to inflammation of the liver, which can lead to liver failure.
Fatigue and headache were the most common side effects reported with Harvoni and Sovaldi. There have been reports of hepatitis B virus reactivation in patients with hepatitis C and hepatitis B. These occurred in patients who were receiving or had received certain hepatitis C medications but were not receiving treatment for hepatitis B.
Gilead Sciences markets Harvoni and Sovaldi.